OECD 422:联合重复剂量毒性研究(w/筛选)
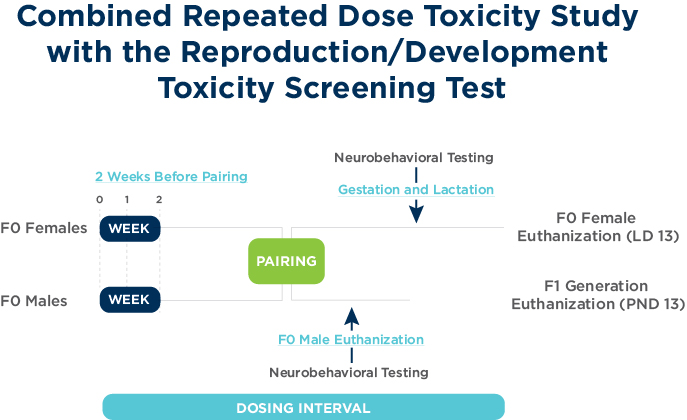
The objectives of the Combined Repeated Dose Toxicity Study with the reproduction/developmental toxicity screening test are to examine chemical exposure on male and female reproductive performance, in addition to the standard requirements for Repeat Dose General Toxicity studies of 4-week duration (OECD 407).
The study design is a combination of the OECD 421 test guideline and the OECD 407 test guideline and therefore adds additional endpoints including clinical pathology, full histopathology and neurobehavioral assessments.
Related Capabilities
-
投掷


