访问监管战略咨询和国家临床看护人(ICCC)服务,使日本新型生物制药产品的最佳产品开发。
在日本制定健全的监管策略
我们投资于您的提交与经验丰富的监管领袖团队的成功。您的意见书由广泛的经验和专业服务在各种治疗区域和适应症中受到支持。
- 访问日本不断发展的监管环境的洞察力
- 实现近同时全球监管批准您的产品
- 获取有关获取索克队指定的建议,加快对再生药物或孤儿药物指定的批准
为日本启用新型生物制药产品的最佳开发
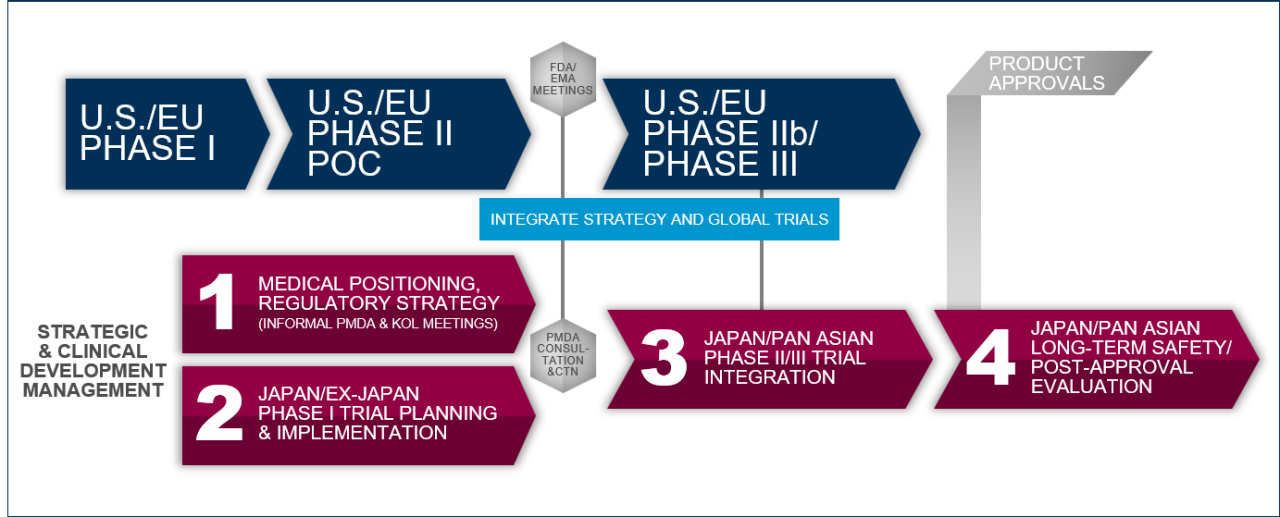
After decades of continuous harmonization of medical practices and with the successful initiative of the Ministry of Health, Labor and Welfare (MHLW) in Japan to create a regulatory framework aligned with the U.S. and EU, Japan has become well integrated into the global drug development landscape.
This steady regulatory reform that made Japan’s integration into U.S. and EU-led drug development programs possible, MHLW and Pharmaceuticals and Medical Devices Agency (PMDA) have now brought forth dynamic initiatives aimed at establishing Japan as a global leader in the development of breakthrough drugs addressing high, unmet medical needs.
Leveraging its multi-disciplinary team of drug development experts, we can not only successfully integrate Japan into global drug development programs but also work with clients to navigate these novel regulatory pathways such as SAKIGAKE, advance approval of regenerative medicines or support a product’s designation as an orphan drug.
让我们帮助您设计开发项目,增加您成功的机会。