你走了多远,取决于你
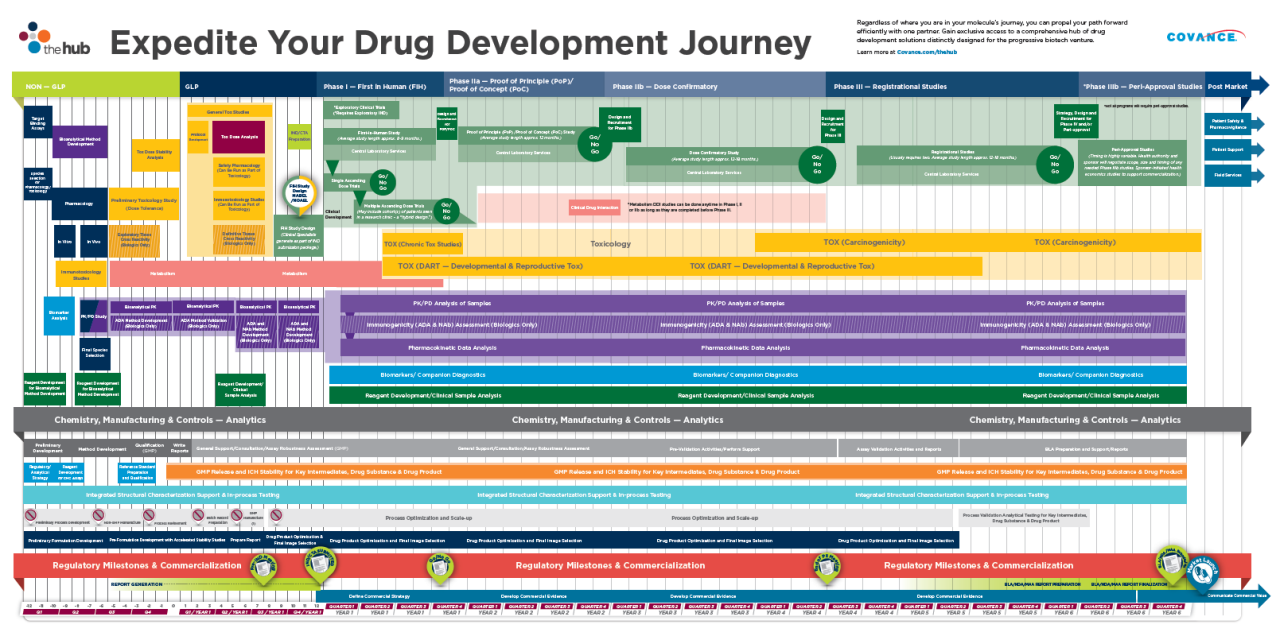
Whether you plan to complete an IND/CTA-enabling program or you need to gain the clinical insight that a first-in-human (FIH) or proof-of-concept (PoC) study can provide, you can enjoy the journey with a dedicated team and a singular, cohesive strategy that transitions seamlessly between nonclinical and clinical development.
Which candidate is best?
获得集成的解决方案,以便快速识别和发展您最好的领导候选人。从早期表征和开发批次制定,对于早期鉴定药理的非GLP筛查,或与毒性相关的问题 - 放心,您将向前移动您的最佳候选人。
IND / CTA-支持非临床评估
Take advantage of the vast knowledge of an expert team who manages drug development programs to support hundreds of regulatory submissions each year. With Early Phase Development Solutions, you seamlessly integrate the complete array of nonclinical services, including lead optimization, safety pharmacology, toxicology, pathology, bioanalytical, drug metabolism and pharmacokinetics, to assure successful design and conduct of your program—all the way through IND/CTA submission and into first in human clinical studies.
首先(FIH)的研究
With Early Phase Development Solutions, you benefit from the retained knowledge from nonclinical study results to move your compound across drug development phases more effectively. The focus will be on two critical aspects of your FIH studies: scientific integrity and human subject safety. As early research continues to demand more complex studies requiring special populations, multiple endpoints and adaptive protocol designs, you’ll gain the advantage through 35+ years of insights and industry-leading human AME expertise.
Proof of Concept (PoC)
Waiting until you have a complete data package before designing your Proof of Concept (PoC) study can waste valuable time. Instead, you’ll enjoy innovative approaches to these shorter, scientifically demanding studies by parallel processing study feasibility and site assessments, incorporating relevant biomarkers and leveraging adaptive trial designs. Increase your clinical ROI by applying the right level of medical, scientific and therapeutic expertise resources and patient stratification strategies to your program.
程序方法 - 为什么它很重要
如何在您的计划上刮起高达30%的人?这是为了简化您的旅程,取出空白,并重新思考风险管理。阅读更多“估计时间对药物发展计划,资产价值和金融公司绩效的影响” whitepaper. What’s more, we can sit down and do an economic valuator session – to estimate the likely time savings for your specific program.