MC38: An immunoresponsive murine tumor model
作者:
雪莉,Barnes博士| Director, Scientific Development
马里兰·富兰克林博士|科学发展副总裁
DATE:
March 2019
结直肠癌是美国第四常见的癌症。结直肠癌是女性癌症相关死亡的第三大原因,男性癌症相关死亡的第二大原因。2019年,估计美国将有超过14.5万个新的结直肠癌病例被确诊,超过5.1万名患者死亡。在过去的几十年里,预防和早期发现的举措,加上治疗方案的改进,已经导致结直肠癌诊断和死亡的减少。这些措施也使五年总生存率提高到64.9%,但那些癌症未被早期发现的患者的生存率急剧下降。[1]For this reason, the development of new treatments for colorectal cancer is a continual need.
The advent of immunotherapy has necessitated同基因小鼠肿瘤模型具有良好的生长动力学和对免疫调节剂的反应,以进一步促进肿瘤的发展immuno-oncologytreatments. One of these colon adenocarcinoma models, MC38, has been characterized by Covance to support development of these agents. MC38 was isolated from a colon tumor in a C57BL/6 mouse following long term exposure to the carcinogen DMH (1,2-dimethylhydrazine dihydrochloride).[2]As described below, MC38 has a favorable response profile to immunomodulatory antibodies suggesting a tumor microenvironment amendable to immune activation. Thus, MC38 is well positioned to be a powerful immuno-oncology model with significant utility in drug development.
MC38 Tumors Following Checkpoint Inhibitor Therapy
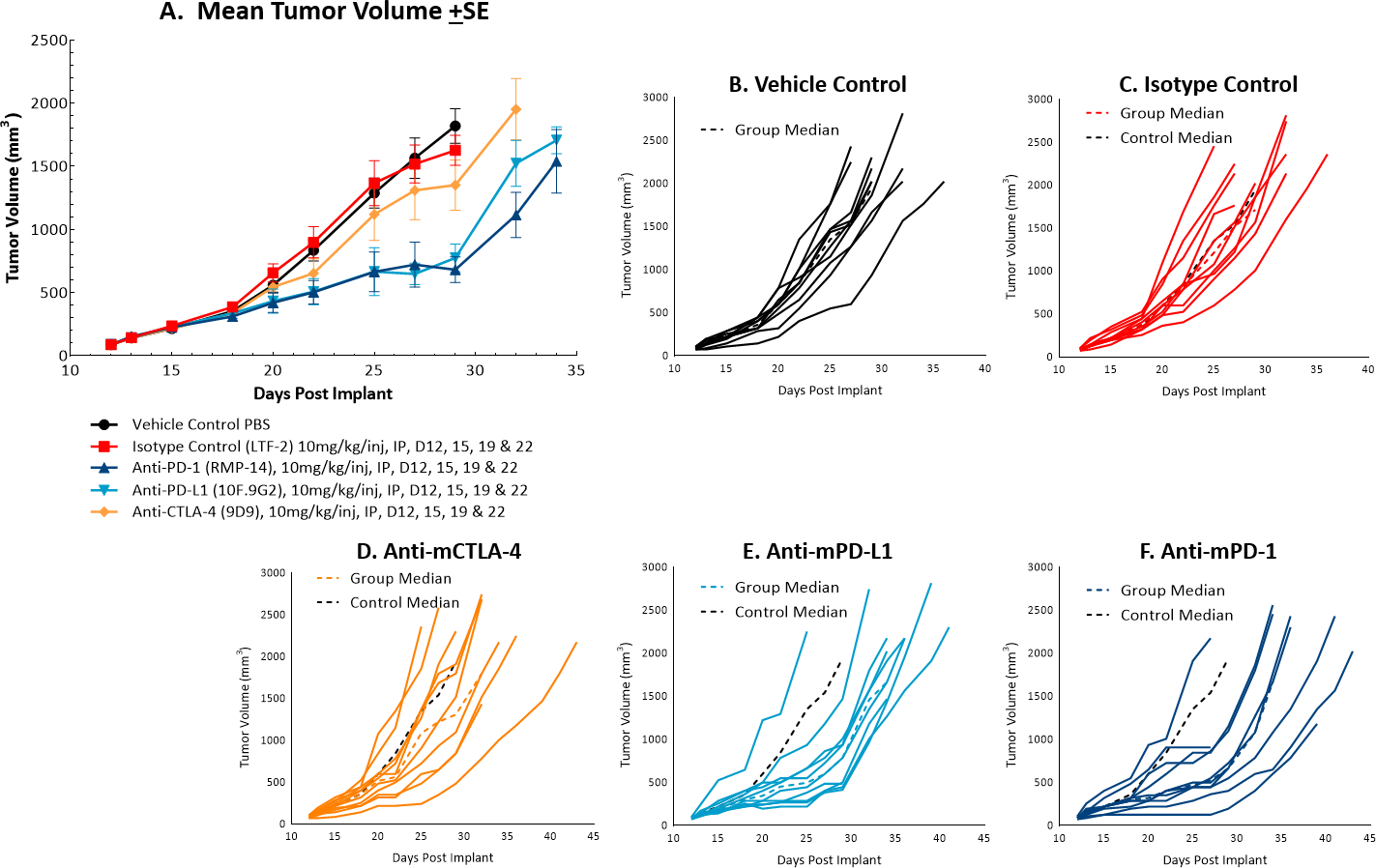
The体内doubling time of subcutaneous MC38 tumors is ~4 days, a moderate growth rate which can facilitate up to a three-week dosing window for test agents to elicit their anti-tumor activity. The model was used in a study to evaluate response to commonly utilized checkpoint inhibitor antibodies. Figure 1 demonstrates mean tumor volumes (A) and individual tumor volumes (B-F) of untreated control tumors compared to those treated with isotype control, anti-mCTLA-4, anti-mPD-L1, or anti-mPD-1. Dosing with all test agents began once tumors were established (~100mm3). Anti-mPD-L1 and anti-mPD-1 demonstrated the most meaningful anti-tumor activities, of the three checkpoint inhibitors, with approximately 6 and 8 days of tumor growth delay on day 22, 40% and 50% putative responders, and an increased time to progression of 32 and 29 days, respectively compared to the untreated control group. The clear effect of these treatments can allow for additive or synergistic improvement in combination with candidate molecules.
MC38 Tumors Following Costimulatory Antibody Therapy
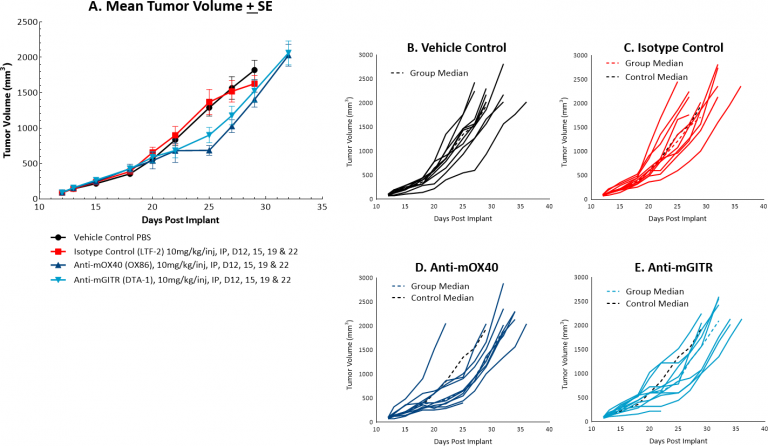
MC38对共刺激分子抗mOX40和抗mGITR的反应也进行了评估,我们发现与图1所示的反应相比,MC38的抗肿瘤活性较弱(见图2A和2B-E)。抗mOX40治疗产生中等的抗肿瘤活性,在第22天ΔT/ΔC中位数为51%,10%假定应答者和4.5天的肿瘤生长延迟。抗mGITR治疗的抗肿瘤活性最低,第22天ΔT/ΔC中位数为72%,假定应答者为20%,肿瘤生长延迟2天。然而,这些药物也显示出活性改善的空间,使MC38成为一种具有广泛免疫调节剂联合治疗的有吸引力的模型。
单用或联合抗mPD-1局部放射治疗MC38肿瘤
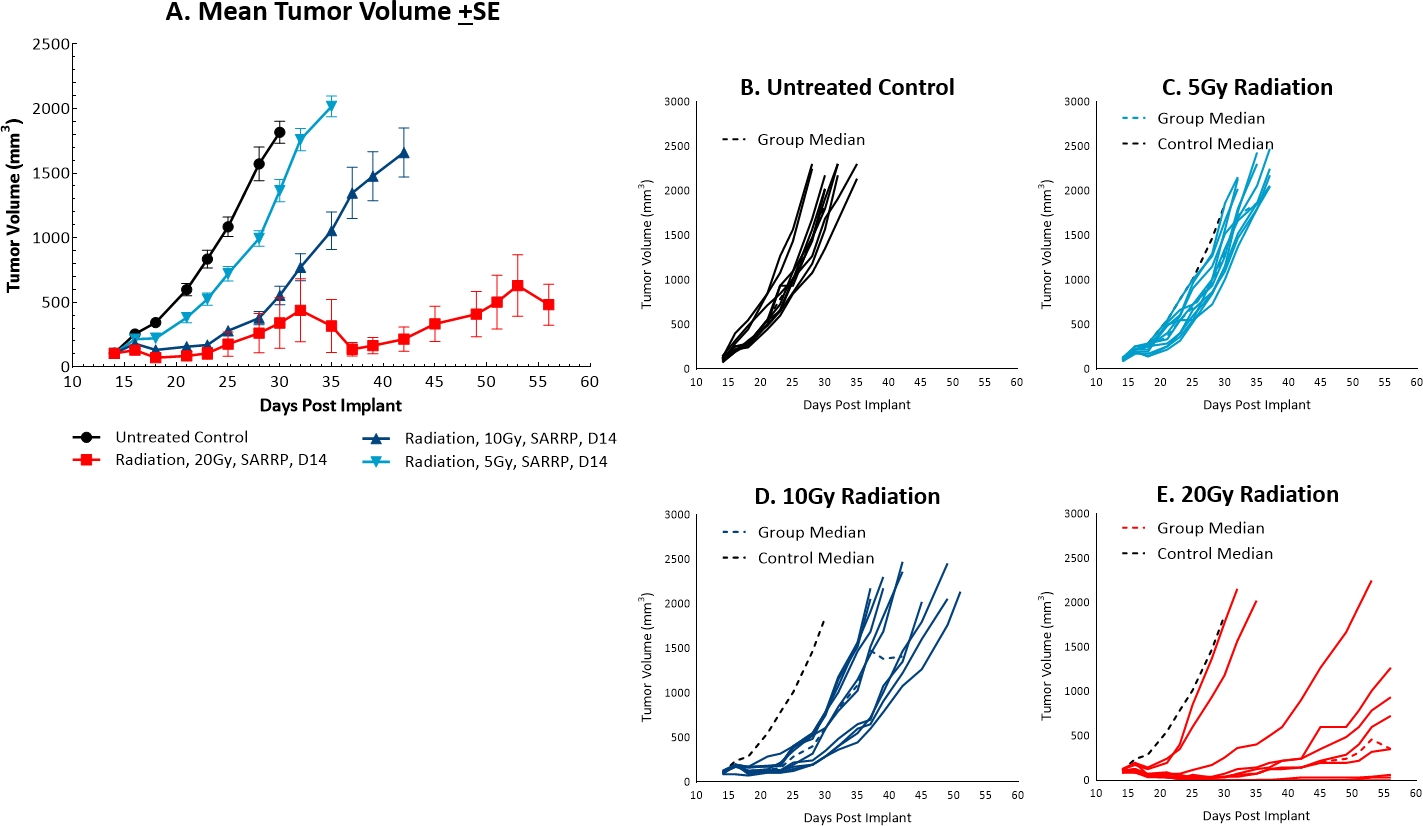
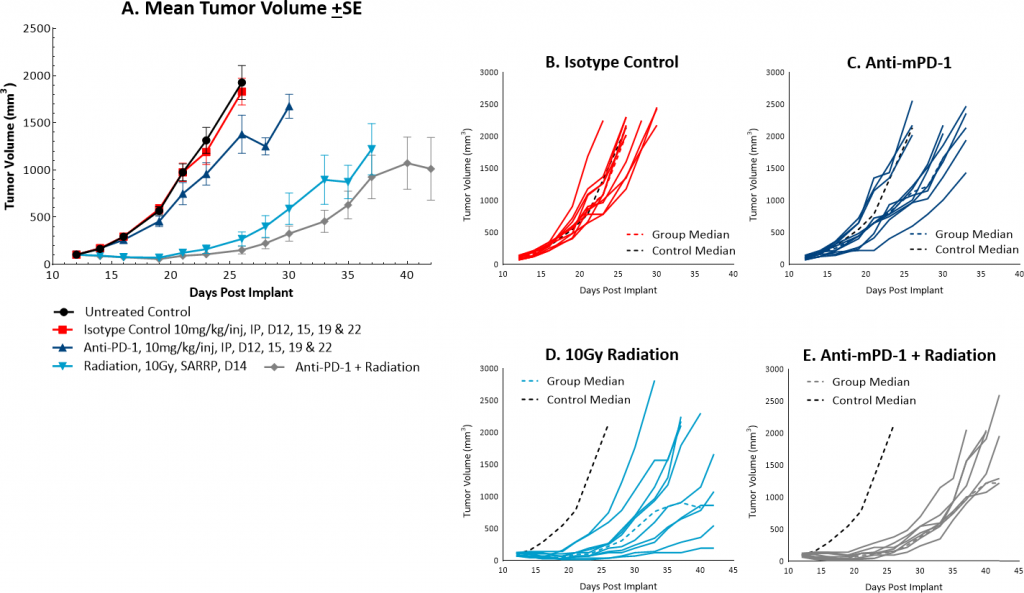
而放射治疗is not commonly utilized in colon cancer, it can be used in certain circumstances such as trying to shrink a tumor prior to surgery or in combination with chemotherapy in patients that may not be healthy enough for surgery. However, in comparison, radiation is used more frequently in rectal cancer. At Covance we utilize the Small Animal Radiation Research Platform (SARRP, Xstrahl) to deliver focal beam radiation to murine models. In the subcutaneousMC38 modelwe tested a single, focal dose of either 5, 10 or 20Gy. We observed a dose response anti-tumor activity following treatment (Figure 3) with 5Gy providing very little activity, 10Gy providing modest activity, and 20Gy providing substantial activity. In follow on work, we tested the combination of 10Gy radiation and anti-mPD-1 in the MC38 tumor model (Figure 4). We found that the combination resulted in improved anti-tumor responses and resulted in 30% tumor free survivors whereas the monotherapies had no tumor free survivors. Therefore, MC38 is also an attractive model to test combination approaches with radiation.
MC38小鼠结肠癌模型可作为一种可靠的临床前免疫肿瘤学模型。我们的数据支持使用这个工具来研究与辐射、检查点抑制剂、共刺激分子或其他新yaboapp体育官网方法的新的治疗组合。拜托联系人Covanceto speak with our scientists about how MC38 or one of our other syngeneic models can be used for your next immuno-oncology study.
[1]Howlader N、Noone AM、Krapcho M、Miller D、Bishop K、Kosary CL、Yu M、Ruhl J、Tatalovich Z、Mariotto A、Lewis DR、Chen HS、Feuer EJ、Cronin KA(编辑)。SEER癌症统计回顾,1975-2014,国家癌症研究所。马里兰州贝塞斯达,https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017.
[2]Corbett TH,Griswold Jr,DP,Roberts JC,Peckham JC,Schabel,Jr,FM(1975)用于化疗分析的小鼠可移植结肠癌发展中的肿瘤诱导关系,以及关于致癌物结构的注释。癌症研究, 35: 2434-2439.