景Medical Device and Diagnostic Solutions
Connected Insights. Expert Delivery. For Every Stage of the Product Life Cycle.

It is an exciting time for medical device and diagnostic development. Innovations such as AI, robotics, mobile/home-health, apps and combination products are transforming healthcare. Advances so forward thinking that they are out-pacing today’s regulatory environment.
Change like this calls for a completely new approach.
Say Helloto a new model that connects insights across your development program interlacing every stage of the product life cycle. Making you smarter, nimbler and more efficient at getting your next medical breakthrough to the patients who need it. Covance Medical Device and Diagnostic Solutions pairs a strategic approach with expert delivery of tests and trials to get the data required. With a specialized team and powerful collection of resources, you gain the knowledge, expertise and tools to bring even your most ground-breaking devices to life.
There for the life cycle of your device
Explore how you can succeed at every stage of development:
A new approach for a changing world
Developing a device is a multi-faceted process with many moving parts.
Regulatory. Quality. Health Economics. Reimbursement. Testing and Trials. Education. Patient Safety. And More.

With Covance, you uniquely attain:
Connected insights
Plan ahead with upfront consulting guidance to create a forward-thinking strategy for your device.
Life cycle integration
Advance your device faster with a cohesive approach to every development stage – preclinical, clinical and post market.
热情的经历
与一个专门的设备专家团队合作,他们对帮助您实现目标的热情。
资源聪明才智
Accelerate recruitment with unparalleled data sources and unique models such as, virtual trials or laboratory testing sites.
Our customers accomplish more. You can too. Allow us to reintroduce ourselves.
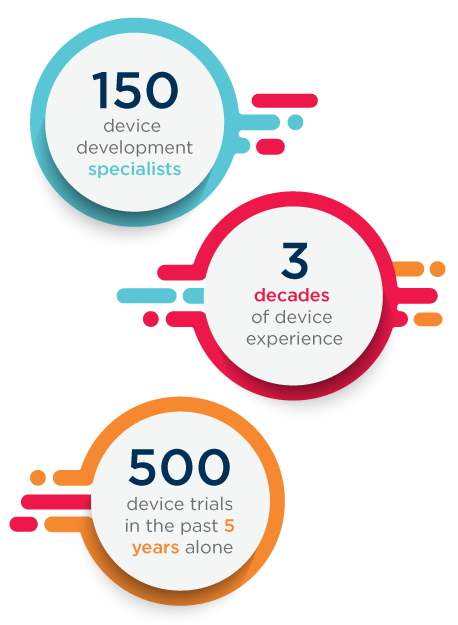
When you choose Covance Medical Device and Diagnostic Solutions, you gain a specialized team of scientific and regulatory professionals with experience in a vast array of clinical areas ready to provide guidance, insights and advice to help you accelerate your device development while avoiding common pitfalls.
Plus, you have access to an even richer, more powerful collective of medical device consulting and global contract research resources – an expanded offering and team made possible through a series of acquisitions to elevate your device development options. Acquisitions in the past five years include: Chiltern, Theorem, PMI, RCRI and ENVIGO.
Go even further by combining the power of our larger organization to meet your goals:
- 景drug development–启用唯一集成的组合产品程序。
- LabCorp diagnostics- 我们的母公司带来了体外诊断开发和实验室检测网站。
New thinking, all within the realms of possibility for today’s changing world.
将您的设备带到生活中。我们能帮你什么吗?
Every medical device and diagnostic product is different. Whether you need a partner for every phase of development or just one study, Covance Medical Device and Diagnostic Solutions can meet the goals for your next breakthrough.
For additional resources visit ourMedical Device and Diagnostics Education Center.